Quanta Receives FDA 510(k) Clearance for the SC+ Hemodialysis System Setting the Stage for US Market Launch
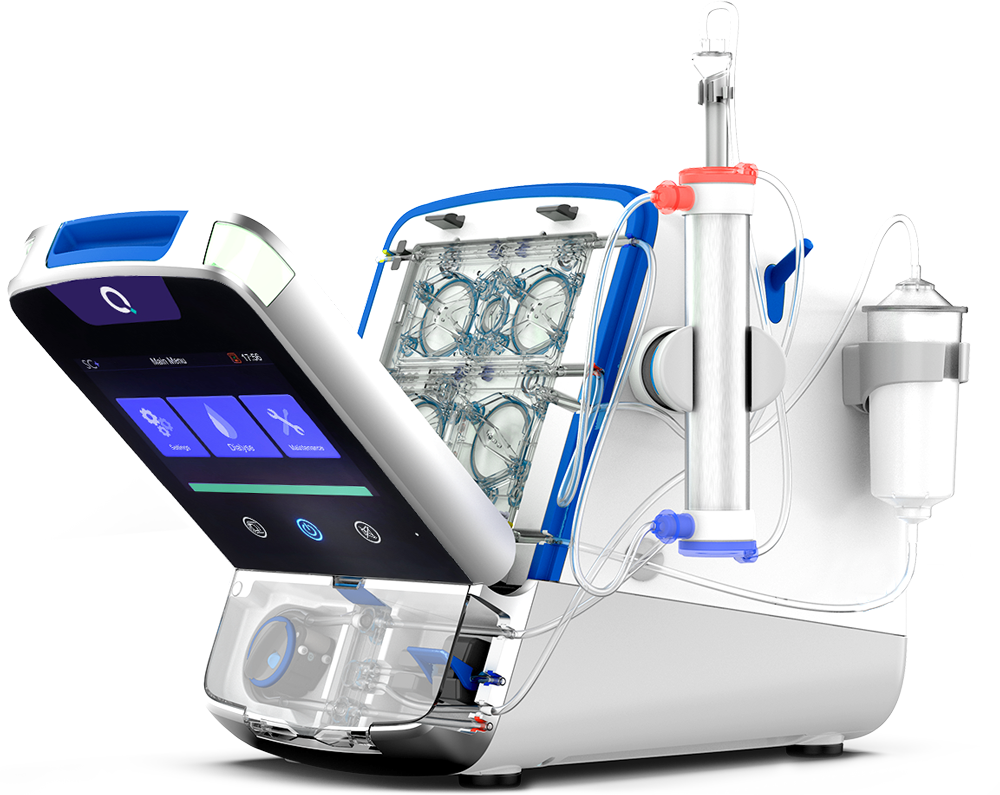
Alcester, Warwickshire, UK, 8 January 2021: Quanta Dialysis Technologies Ltd (“Quanta” or “the Company”), a British medical technology pioneer developing innovative dialysis products and services for the global market, today announces it has received 510(k) clearance from the US Food and Drug Administration (“FDA”) to market its small and simple, high performance hemodialysis system SC+. This critical milestone enables Quanta to bring to the American market its portable device that can provide a dialysis dose equivalent to today’s standard of care, but in a compact, easy-to-use format suitable for a range of care settings.
The SC+ System in the US
There are more than half a million patients requiring hemodialysis in the United States. The vast majority of these patients receive dialysis three-times-a-week, with a minority receiving more frequent therapy. Unlike other portable hemodialysis systems, SC+ can deliver the higher dialysate flow rates typically used to provide conventional three-times-a-week prescriptions, while also offering the flexibility for more frequent, longer and gentler treatments tailored to patients’ needs. The small, lightweight design of SC+ makes the device portable, allowing for dialysis treatments to be brought to patients, and the simple, intuitive user interface makes dialysis accessible to a broad range of healthcare professionals.
In preparation for commercial launch, Quanta continues to develop its US team and operations. Following the appointment earlier this year of the Company’s President of North America, John Lipman, Quanta has also recently hired a Head of Chronic Sales and a Head of Clinical Support, while also building a dedicated US-based team that includes training, customer care and technical staff to support the Company’s customers and users.
To complement SC+, Quanta will introduce a suite of related products and services. This includes an optional, portable water purification module that will enable SC+ to be easily moved around and to be operated in a wide range of settings, both with and without a centralized water system, and a secure, cloud-based digital health offering that will simplify and automate treatment data capture and reporting, negating the need to create and store manual records.
Following extensive clinical piloting with the National Health Service (NHS) in England, SC+ became commercially available in the UK last year, playing a vital role in supporting the increased need for more simple, flexible dialysis devices in the hospital and the home, particularly during the rise of the COVID-19 pandemic. As reported in a recently published study (Komenda et al, Kidney Medicine 2020), SC+ combines the power and performance of larger conventional dialysis machines with the convenience of a compact, portable, user friendly device, making SC+ a much-needed addition to existing dialysis treatment options.